FDA launches look into safety of drug coated stents
Go Deeper.
Create an account or log in to save stories.
Like this?
Thanks for liking this story! We have added it to a list of your favorite stories.

Stents are tiny wire mesh devices that prop open clogged heart arteries. Older versions are made with bare metal. For the last three years a new model, with a drug coating, has been on the market. The drug-coated stents have become so popular, nearly 90-percent of all stent procedures in the U.S. use the drug-coated stent. Last year approximately 990,000 heart patients received the devices.
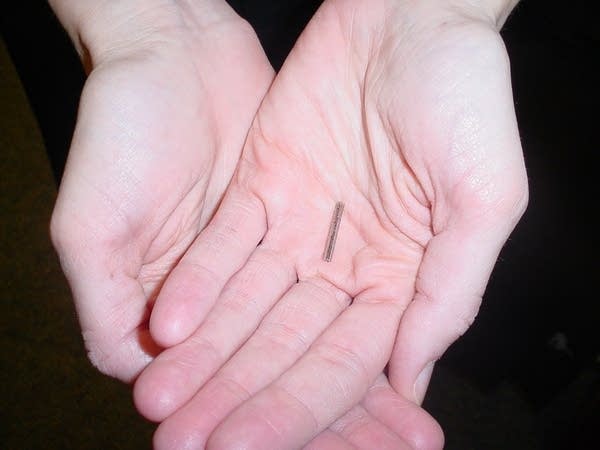
But there may be a problem with these drug-coated stents. Studies have shown they increase the risk of life-threatening blood clots when compared to the older bare metal stents.
The drug-coated stents have been celebrated because they help prevent excess scar tissue from forming around the stent and narrowing the artery again. But cardiologists say a small amount of scar tissue is actually a good thing, because it helps keep the stent smooth inside the artery wall allowing the blood to flow freely. In some patients with drug-coated stents there may not enough scar tissue to keep the stent positioned smoothly against the artery wall and that may lead to some serious problems.
The findings have rattled cardiologists throughout the U.S. But the reports haven't stopped cardiologist Gladwin Das from using drug-coated stents in most of his cardiac patients.
Turn Up Your Support
MPR News helps you turn down the noise and build shared understanding. Turn up your support for this public resource and keep trusted journalism accessible to all.
"We're gonna equalize you. Hold on for a second. You can go AP cranial please," Das says while studying a pulsating image of two drug-coated stents projected on a monitor at the University of Minnesota Medical Center, Fairview in Minneapolis.

The stents belong to a female patient lying on the table in front of him. She's come in to the hospital with chest pain and wonders if there's a problem with her stents. Das inspects the devices and determines they're working just fine.
"I think the concern about safety has been blown out of proportion. We need to be concerned about the safety as far as any new device is concerned. But I don't think it's born out by a careful look at the data."
Das is more inclined to believe findings from a recent review of patient data from Cordis, the company that manufactures a drug-coated stent for Johnson and Johnson. After the safety questions arose, a panel of medical experts examined the company's patient data. Das says the review, by independent researchers, found that the risk of clotting in the company's drug-eluting stents is about the same as bare metal stents.
"If you look at the study which came out from Cordis within the first 30 days, .5 percent which is one in 200 patients with a drug-coated stent have their stents clotting off, or having a heart attack...It's .3 percent in these same studies in the patients who got bare metal stents. And this difference is not significantly different."

Das says the same Cordis study shows that four years after stent implantation, the clotting rate is still about the same for drug-coated and bare metal stents.
But for each study that shows a minimal clotting difference between the stents, there are many others that suggest the difference in clotting rates is significant. A recent meta-analysis of 14 studies calculated that the risk of blood clots was four to five times higher in patients who receive a drug-coated stent.
These mixed messages have left stent patients like Stefan Silverman wondering what to make of it all.
Silverman received his drug-eluting stents two years ago after experiencing intense arm pain while driving his car to work. When he went to the doctor he was relieved to learn that he wasn't having a heart attack. But he discovered his risk for one was high. One of his arteries was 90-percent clogged and needed to be re-opened quickly.
Today the 56-year-old husband and father of two is pain-free and doing normal things, like seeing his daughter Kira off to school.
Until recently Silverman hadn't given his stents much thought. But after reading a report on the safety questions in his local newspaper, he got very concerned.
"I read that and said gee, this is me. This is the procedure that I had. I have two of them and you know what do I need to know about this?"
Reading the article made Silverman realize that he didn't know much about his stents. He says at the time of his procedure he didn't even think to ask whether he had other options.
"When the doctor says, this is what you need, I looked up from the table and said, well if that's what you think. What else can I say?"
But now Silverman wonders if he would have been better off with bare metal stents. He plans to ask his doctor during his next check up in January.
Dr. Timothy Henry didn't treat Silverman. But the Minneapolis Heart Institute cardiologist suspects Silverman received the appropriate stent based on his apparent good health today.
"He's two years out and has not had a recurrence and if he'd got a bare metal stent he would have had a 25 to 30-percent chance of having had a second procedure. He's a clear cut example of the benefits of drug-eluting stents and why we put them in," he says.

The recurrence Henry describes is a condition called restinosis. It's a re-narrowing of the artery. Remember how some scar tissue on a stent is a good thing? In this case, too much scar tissue forms, clogging the artery up again. Usually restinosis doesn't cause a serious problem like a heart attack. But it can cause significant discomfort and expense if patients need to undergo a second procedure to re-open the artery. Given a choice, Henry believes most patients would rather have a drug-coated stent to avoid the possibility of restinosis.
But reports suggesting that drug-coated stents have a higher blood clotting risk do concern Henry. He thinks the safety questions will cause cardiologists to be more cautious about using the devices, especially in high-risk patients who have already had a heart attack or have other complicating medical conditions.
"I think whereas the United States went from zero percent drug-eluting stents up to 90-plus-percent very quickly. I think what's generally happening is now is we're slowing going backwards on that and we'll come to a comfortable number. What will that number be? It's hard to know."
Henry also thinks drugs will become a much more important part of long-term stent therapy, a view that may be shared by regulators. Just this week the FDA said on its Web site that it's safety panel will consider whether patients with drug-coated stents may need to use blood-thinning drugs like aspirin and Clopidogrel, also known by the brand-name Plavix, for much longer periods of time. Currently patients are advised to take the drugs for three to six months. But recent studies have shown that patients who take them for at least a year or longer appear to have a much lower clotting risk associated with their drug-coated stents.
In the next year, the FDA is also expected to recommend new guidelines that could extend the research period for companies making the next generation of drug-coated stents.
Stents are a multi-billion dollar industry in the U.S. and a significant part of the Minnesota economy.
Nearly 3,300 people work for Boston Scientific at its stent manufacturing facilities in Plymouth and Maple Grove. Another 140 are employed at Eden Prairie-based SurModics, which makes the drug-coating for Johnson and Johnson stents.
Medtronic, which is based in Fridley, employs about 5000 workers in its vascular division facilities in California and Ireland. Medtronic doesn't have FDA permission yet to sell its drug-coated stent in the U.S. But the company says it hopes to receive FDA approval by mid 2007.




